Our Pipeline
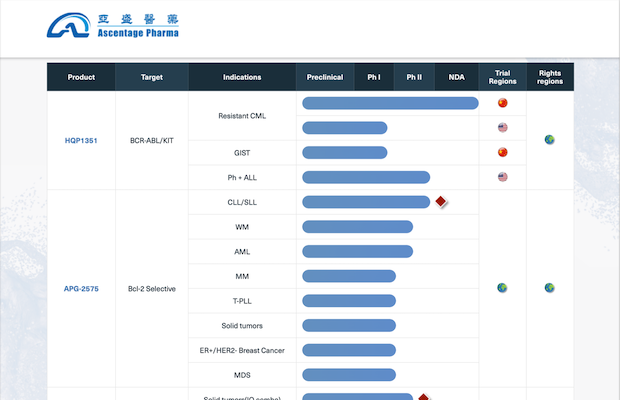
Website: https://ascentage.com/science/pipeline/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: MDM2-p53 Inhibitor
Treatment: In clinic, Alrizomadlin monotherapy and combination (i.e., PD-1 blockade) therapy are being developed for the treatment of various solid tumors and hematologic malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), T-cell prolymphocytic leukemia (T-PLL), and malignant peripheral-nerve sheath tumor (MPNST), advanced solid tumors, as well as other indications. Alrizomadlin has been granted Orphan Drug Designations by the U.S. FDA for the treatment of gastric cancer, soft-tissue sarcoma, AML, retinoblastoma, IIB-IV melanoma, and neuroblastoma.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCL-2 Selective Inhibitor
Treatment: Currently, APG-2575 is under clinical development in patients with hematologic malignancies and breast cancer globally. Lisaftoclax is in development as a single agent or as a component of combination therapy in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), acute myeloid leukemia (AML) and Waldenström macroglobulinemia (WM) and has potential for development in additional indications. Lisaftoclax has been granted Orphan Drug Designations by the U.S. FDA for the treatment of chronic lymphocytic leukemia, Waldenström macroglobulinemia, multiple myeloma, AML and FL. Proof-of-concept has been achieved in the patients with CLL.
Website: https://ascentage.com/science/research-development/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
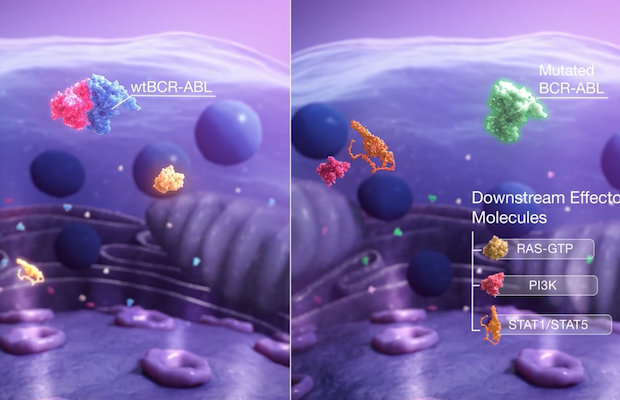
Class: BCR-ABL/KIT Kinase Inhibitor
Treatment: Olverembatinib is also under the clinical development for the treatment of gastrointestinal stromal tumors (GISTs) owing to its potent activity in inhibiting c-KIT oncogene. The latter is the crucial driver in GISTs. Further, by targeting FLT3, olverembatinib has demonstrated potent antitumor activity in preclinical models of FLT3 mutant acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Olverembatinib has been granted Fast Track and Orphan Drug Designations by the U.S. FDA for the treatment of patients with CML and has received the priority review for its New Drug Application from the China regulatory authorities.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
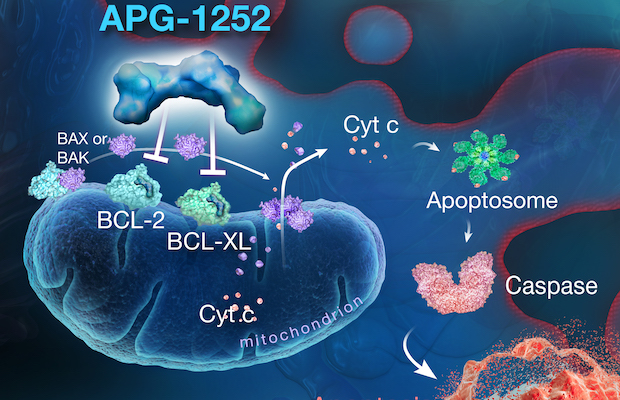
Class: Dual BCL-2/BCL-xL Inhibitor
Treatment: Pelcitoclax combination treatments are being evaluated for the treatment of small-cell and non-small-cell lung cancer and has potential in the treatment of specific lymphomas, gastrointestinal tumors, and myelofibrosis.
Website: https://ascentage.com/science/research-development/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
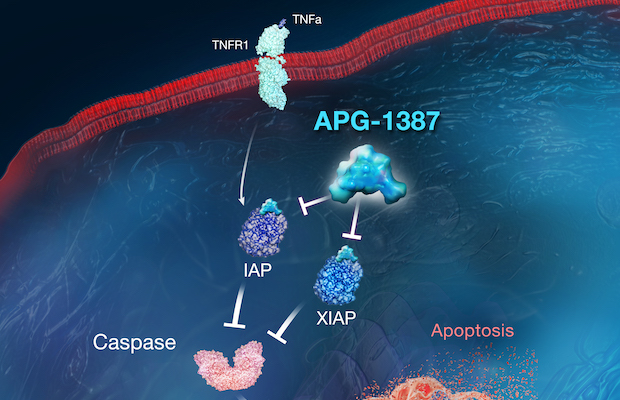
Class: SMAC Mimetic Dimer IAP Antagonist
Treatment: This candidate is being evaluated in a combination approach for the treatment of advanced pancreatic cancer. In addition, APG-1387 combination therapy is being evaluated for the treatment of chronic hepatitis B infection.
Website: https://ascentage.com/science/research-development/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
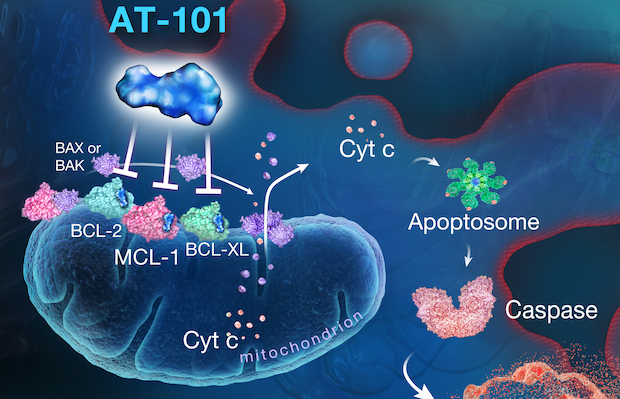
Class: a small molecule oral inhibitor for the Bcl-2 family
Treatment: This candidate has activity alone and in combination with docetaxel (Taxotere)® and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC).
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
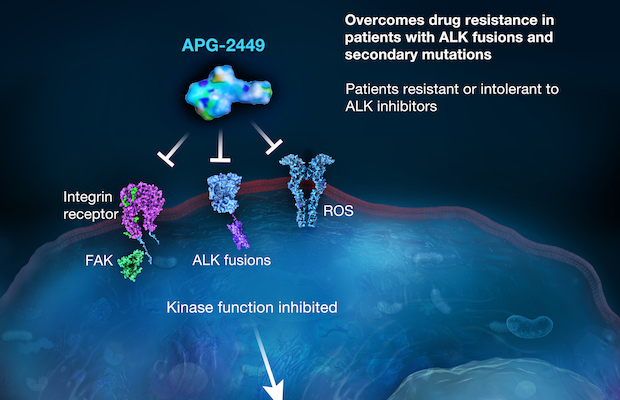
Class: FAK/ALK/ROS1 Tyrosine Kinase Inhibitor (TKI)
Treatment: In preclinical models of ALK-positive NSCLC, APG-2449 has demonstrated antitumor activity and the ability to overcome resistant mutations the first generation and second generation ALK inhibitors, including G1202R mutations. In addition, APG-2449 single agent and combination therapy effectively inhibits tumor growth of ALK-positive neuroblastoma in preclinical setting. Through targeting FAK signaling pathway, APG-2449 demonstrates synergistic or enhanced antitumor activity in combination therapy in the preclinical tumor models of mesothelioma, EGFR mutant NSCLC, and ovarian cancer. APG-2449 is currently in clinical development for treatment of patients with NSCLC failed to respond to treatment with earlier-generation ALK inhibitors.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
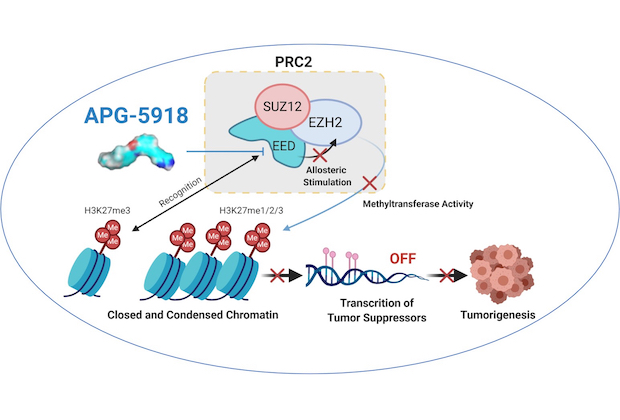
Class: a small molecule oral inhibitor for the Bcl-2 family
Treatment: APG-5918 demonstrates excellent antitumor activity in preclinical xenograft models of multiple blood cancers and solid tumors including EZH2 mutant diffuse large B cell lymphoma (DLBCL) and SMARCB1-deficient malignant rhabdoid tumor. APG-5918 is currently under preclinical development for IND filing.
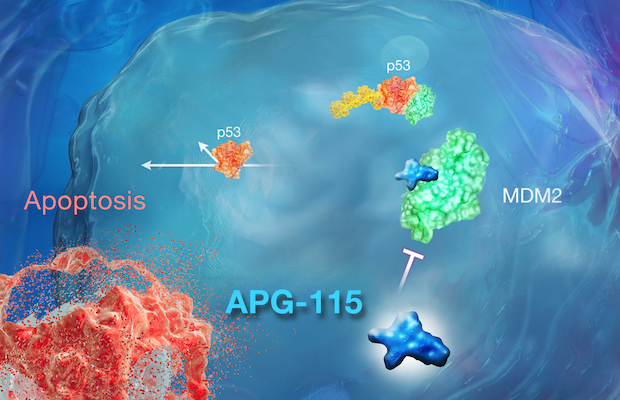
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: MDM2-p53 Inhibitor
Treatment: In clinic, Alrizomadlin monotherapy and combination (i.e., PD-1 blockade) therapy are being developed for the treatment of various solid tumors and hematologic malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), T-cell prolymphocytic leukemia (T-PLL), and malignant peripheral-nerve sheath tumor (MPNST), advanced solid tumors, as well as other indications. Alrizomadlin has been granted Orphan Drug Designations by the U.S. FDA for the treatment of gastric cancer, soft-tissue sarcoma, AML, retinoblastoma, IIB-IV melanoma, and neuroblastoma.
Website: https://ascentage.com/science/research-development/
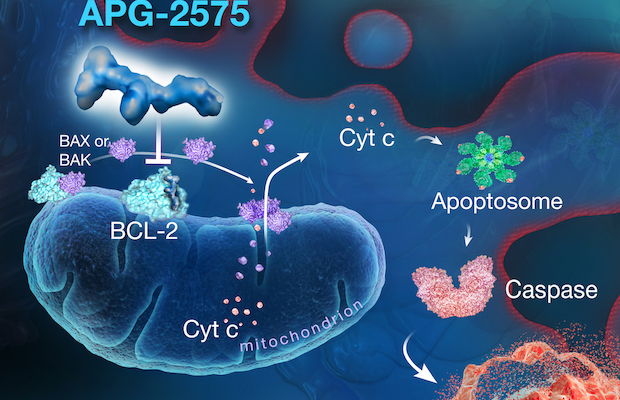
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCL-2 Selective Inhibitor
Treatment: Currently, APG-2575 is under clinical development in patients with hematologic malignancies and breast cancer globally. Lisaftoclax is in development as a single agent or as a component of combination therapy in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), acute myeloid leukemia (AML) and Waldenström macroglobulinemia (WM) and has potential for development in additional indications. Lisaftoclax has been granted Orphan Drug Designations by the U.S. FDA for the treatment of chronic lymphocytic leukemia, Waldenström macroglobulinemia, multiple myeloma, AML and follicular lymphoma (FL). Proof-of-concept has been achieved in the patients with CLL.
Website: https://ascentage.com/science/research-development/
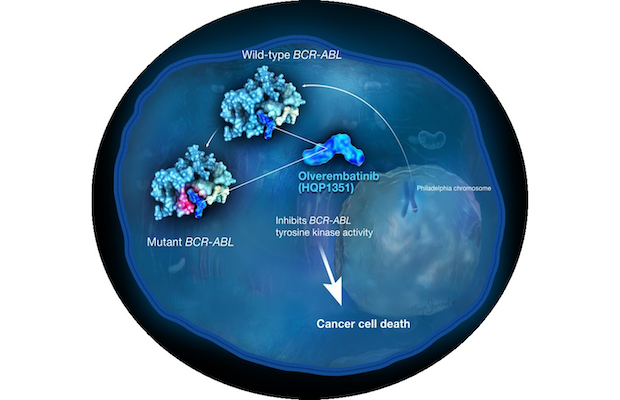
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCR-ABL/KIT Kinase Inhibitor
Treatment: Olverembatinib is also under the clinical development for the treatment of gastrointestinal stromal tumors (GISTs) owing to its potent activity in inhibiting c-KIT oncogene. The latter is the crucial driver in GISTs. Further, by targeting FLT3, olverembatinib has demonstrated potent antitumor activity in preclinical models of FLT3 mutant acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Olverembatinib has been granted Fast Track and Orphan Drug Designations by the U.S. FDA for the treatment of patients with CML and has received the priority review for its New Drug Application from the China regulatory authorities.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/

Website: https://ascentage.com/science/pipeline/
©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: MDM2-p53 Inhibitor
Treatment: In clinic, Alrizomadlin monotherapy and combination (i.e., PD-1 blockade) therapy are being developed for the treatment of various solid tumors and hematologic malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), T-cell prolymphocytic leukemia (T-PLL), and malignant peripheral-nerve sheath tumor (MPNST), advanced solid tumors, as well as other indications. Alrizomadlin has been granted Orphan Drug Designations by the U.S. FDA for the treatment of gastric cancer, soft-tissue sarcoma, AML, retinoblastoma, IIB-IV melanoma, and neuroblastoma.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCL-2 Selective Inhibitor
Treatment: Currently, APG-2575 is under clinical development in patients with hematologic malignancies and breast cancer globally. Lisaftoclax is in development as a single agent or as a component of combination therapy in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), acute myeloid leukemia (AML) and Waldenström macroglobulinemia (WM) and has potential for development in additional indications. Lisaftoclax has been granted Orphan Drug Designations by the U.S. FDA for the treatment of chronic lymphocytic leukemia, Waldenström macroglobulinemia, multiple myeloma, AML and FL. Proof-of-concept has been achieved in the patients with CLL.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCR-ABL/KIT Kinase Inhibitor
Treatment: Olverembatinib is also under the clinical development for the treatment of gastrointestinal stromal tumors (GISTs) owing to its potent activity in inhibiting c-KIT oncogene. The latter is the crucial driver in GISTs. Further, by targeting FLT3, olverembatinib has demonstrated potent antitumor activity in preclinical models of FLT3 mutant acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Olverembatinib has been granted Fast Track and Orphan Drug Designations by the U.S. FDA for the treatment of patients with CML and has received the priority review for its New Drug Application from the China regulatory authorities.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: Dual BCL-2/BCL-xL Inhibitor
Treatment: Pelcitoclax combination treatments are being evaluated for the treatment of small-cell and non-small-cell lung cancer and has potential in the treatment of specific lymphomas, gastrointestinal tumors, and myelofibrosis.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: SMAC Mimetic Dimer IAP Antagonist
Treatment: This candidate is being evaluated in a combination approach for the treatment of advanced pancreatic cancer. In addition, APG-1387 combination therapy is being evaluated for the treatment of chronic hepatitis B infection.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: a small molecule oral inhibitor for the Bcl-2 family
Treatment: This candidate has activity alone and in combination with docetaxel (Taxotere)® and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC).

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: FAK/ALK/ROS1 Tyrosine Kinase Inhibitor (TKI)
Treatment: In preclinical models of ALK-positive NSCLC, APG-2449 has demonstrated antitumor activity and the ability to overcome resistant mutations the first generation and second generation ALK inhibitors, including G1202R mutations. In addition, APG-2449 single agent and combination therapy effectively inhibits tumor growth of ALK-positive neuroblastoma in preclinical setting. Through targeting FAK signaling pathway, APG-2449 demonstrates synergistic or enhanced antitumor activity in combination therapy in the preclinical tumor models of mesothelioma, EGFR mutant NSCLC, and ovarian cancer. APG-2449 is currently in clinical development for treatment of patients with NSCLC failed to respond to treatment with earlier-generation ALK inhibitors.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: a small molecule oral inhibitor for the Bcl-2 family
Treatment: APG-5918 demonstrates excellent antitumor activity in preclinical xenograft models of multiple blood cancers and solid tumors including EZH2 mutant diffuse large B cell lymphoma (DLBCL) and SMARCB1-deficient malignant rhabdoid tumor. APG-5918 is currently under preclinical development for IND filing.

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: MDM2-p53 Inhibitor
Treatment: In clinic, Alrizomadlin monotherapy and combination (i.e., PD-1 blockade) therapy are being developed for the treatment of various solid tumors and hematologic malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), T-cell prolymphocytic leukemia (T-PLL), and malignant peripheral-nerve sheath tumor (MPNST), advanced solid tumors, as well as other indications. Alrizomadlin has been granted Orphan Drug Designations by the U.S. FDA for the treatment of gastric cancer, soft-tissue sarcoma, AML, retinoblastoma, IIB-IV melanoma, and neuroblastoma.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCL-2 Selective Inhibitor
Treatment: Currently, APG-2575 is under clinical development in patients with hematologic malignancies and breast cancer globally. Lisaftoclax is in development as a single agent or as a component of combination therapy in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), acute myeloid leukemia (AML) and Waldenström macroglobulinemia (WM) and has potential for development in additional indications. Lisaftoclax has been granted Orphan Drug Designations by the U.S. FDA for the treatment of chronic lymphocytic leukemia, Waldenström macroglobulinemia, multiple myeloma, AML and follicular lymphoma (FL). Proof-of-concept has been achieved in the patients with CLL.
Website: https://ascentage.com/science/research-development/

©2022 Ascentage Pharma (Suzhou) Co., Ltd. and Ascentage Pharma Group Corp Ltd.
Class: BCR-ABL/KIT Kinase Inhibitor
Treatment: Olverembatinib is also under the clinical development for the treatment of gastrointestinal stromal tumors (GISTs) owing to its potent activity in inhibiting c-KIT oncogene. The latter is the crucial driver in GISTs. Further, by targeting FLT3, olverembatinib has demonstrated potent antitumor activity in preclinical models of FLT3 mutant acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Olverembatinib has been granted Fast Track and Orphan Drug Designations by the U.S. FDA for the treatment of patients with CML and has received the priority review for its New Drug Application from the China regulatory authorities.
Website: https://ascentage.com/science/research-development/cell-signaling-inhibitors/